Entwicklung von Reaktionsmechanismen unter Parameteridentifikation von Subsystemen
- Contact:
Dr.-Ing. Nadia Sebbar
- Project Group:
Prof. Dr.-Ing. D. Trimis
Development of reaction mechanisms with parameter identification of subsystems
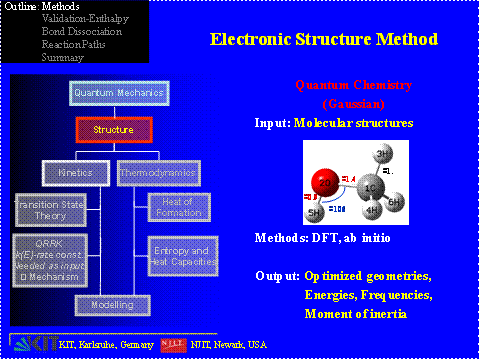
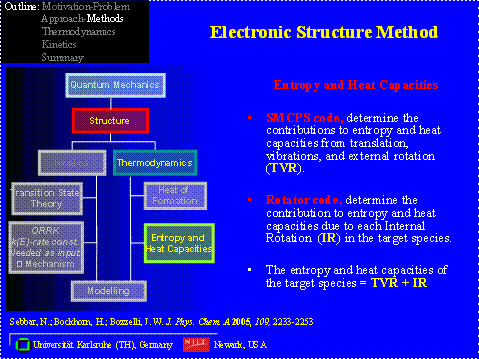
Computational Method All of the calculations are performed using the Gaussian 03 program suite, which calculates the total energies, the frequencies and moment of inertia. The determination of the entropies and heat capacities are calculated with the help of two codes: SMCPS and ROTATOR.
Main Objectives
Application of computational quantum chemistry (ab inito and DFT calculations) is used to estimation of thermochemical properties and kinetic parameters of small and large species, including biochemicals, large aromatic intermediates in soot formation, carbon nanotubes and many other systems.
Introduction
Aromatic and poly-aromatic compounds are important components of fuels. They are also formed in pyrolysis reactions and in fuel rich regions of flames and other thermal systems, where they are considered important precursors and intermediates in soot and PAH formation. The decomposition products of these species in combustion and oxidation reactions involve the incorporation of oxygen through reactions with oxygenated radicals and with molecular oxygen forming species like hydroperoxides or unsaturated oxy-hydrocarbon.
This work involves the development of an extensive thermochemical property data base on unsaturated (olefinic, acetylenic and aromatic) oxygenated hydrocarbon chemical species: enthalpy of formation, bond energies, standard entropy and heat capacity data as a function of temperature are calculated. These data serves the modelling communities in the area of atmospheric chemistry, combustion, industrial processes relating to synthesis and use in synthesis of hydroperoxides, peroxides and peroxy radical species. These parameters are available over a wide range of temperature (200 to over 5000 K) which can be applied to all of the above areas.
Peroxides and peroxy species are perhaps the most important intermediates in all low and moderate (1200 – 1300 K) combustion and in atmospheric photochemical oxidation processes of hydrocarbons and derivatives. Examples of the importance of these peroxides and peroxy intermediates include: control of self ignition in internal combustion engines (knock in spark ignited engines plus fuel ignition in diesels and in the new and upcoming HCCI engines. The peroxy radical reaction chemistry also controls the negative temperature behavior of hydrocarbons where there is a competition between the complex chain branching paths and termination reactions. Alkyl hydroperoxide species are also important in limiting soot formation and in soot burn-out in hydrocarbon pyrolysis and combustion.
A very significant quantity of thermochemical parameters are generated for stable molecules, intermediate radicals and transition state structures of oxygenated hydrocarbon molecules, particularly unsaturated and aromatic carbon – oxygen systems.
Example of Work
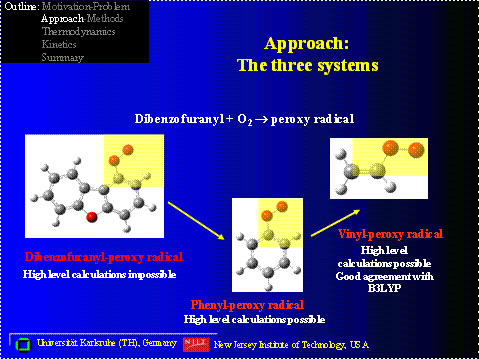
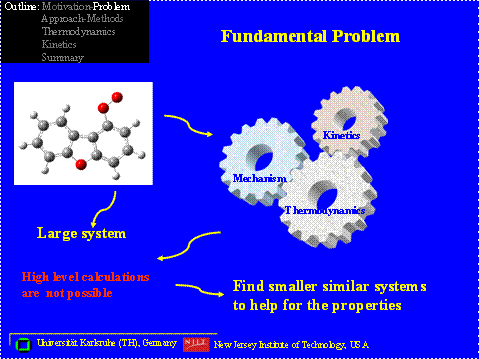
One example of oxidative destructive in aromatics involves conversion of dibenzofurans and / or dioxins in combustion systems. The destruction of the aromatic moiety often starts with loss of a phenyl hydrogen, through abstraction by radical pool species, such as H, O, OH, or Cl, forming a phenyl or benzofuranyl radical. This occurs even at moderate temperatures in downstream zones of an incinerator. The phenyl radicals will rapidly react with molecular oxygen in the combustion environment to form an energized (phenyl peroxy) adduct, which can undergo further reaction through several pathways. Figure 1 illustrates some of the intermediate products from decomposition reactions (beta scission (unzipping) and oxidation) of a benzofuran di-aldehyde formal radical, which is estimated is the major product from reaction of molecular oxygen with a dibenzofuran – phenyl radical. The decomposition reaction results in a number of intermediates and radical products, which do not have thermochemistry or groups for use in group additivity estimations. Knowledge of the thermochemical properties of these species is important to understanding the decomposition and oxidation pathways of these intermediates, which result from initial oxidative ring opening of the aromatics.
For such larger molecules, high level, calculation techniques are computationally expensive or not possible. Density Functional Theory may be one of the few applicable calculation methods for these large molecules systems.
Computational Method
All of the calculations are performed using the Gaussian 03 program suite, which calculates the total energies, the frequencies and moment of inertia. The determination of the entropies and heat capacities are calculated with the help of two codes: SMCPS and ROTATOR.